A Doctor, A Patient, A Breakthrough
Imagine waking up one morning with searing pain in your head and around your eye, your vision threatened by escalating infection from the skin into the eye, and knowing that conventional treatment might not work fast enough to save it. That was my reality.
At 75 years old, I faced herpes zoster ophthalmicus (HZO)—a severe form of shingles that attacks the eye. The swelling around my orbit was so intense that I knew I had to act fast (see picture below and video).
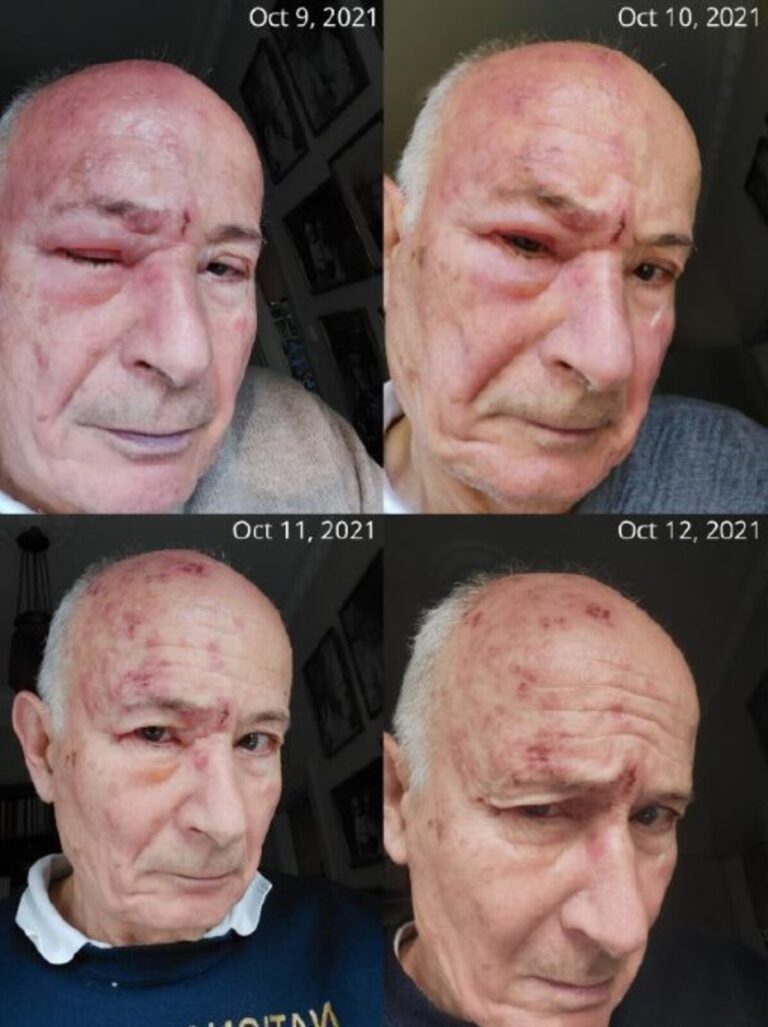
Standard antiviral treatment, acyclovir, typically takes two weeks to heal herpes zoster. In older patients, however, as virus-specific host immunity declines with age acyclovir often fails entirely. I had only one chance to protect my eyesight.
As both a medical doctor and researcher, I decided to complement acyclovir with an experimental oral therapy—a harmless avian vaccine virus known as IBDV, which is developed by my company, HepC Inc. This virus, though non-pathogenic, has the ability to strongly stimulate the body’s natural antiviral defenses. The result? My recovery was astonishing—within just a few days, the painful blisters crusted over, a visible sign of healing that normally takes two weeks on average.
Why This Matters for Patients, Medical & Research Communities and Healthcare
- Cut recovery time dramatically—real hope for faster healing! Faster healing means less pain, fewer complications, and lower healthcare costs. Older patients—who often struggle with waning antiviral defense—could finally have a reliable treatment option.
- Potential paradigm shift in antiviral therapy—help validate this approach!
- Cost-effective strategy to reduce complications & hospitalizations! Insurance companies could see reducing long-term medical costs via add-on therapy.
A Call to Action
This discovery, which has already been demonstrated to be safe and effective against several viral infections (hepatitis A, B, and C viruses, SARS-CoV-2 virus and herpes zoster virus), could change the way we treat viral infections. But we need funding to conduct clinical trials and bring this therapy to the world. Your support—whether as a patient, an advocate, or an investor—can help us validate this breakthrough and make rapid recovery a reality.
Support the Future of Antiviral Therapy
A Paradigm Shift in Fighting Viruses
In a world still recovering from the devastating effects of COVID-19, it is clear that traditional antiviral approaches alone are not enough. Our groundbreaking research introduces a scalable, disruptive therapy—using a harmless avian vaccine virus to control infections. This paradigm-changing innovation could revolutionize how we combat viral diseases and prepare humanity for future pandemics.
As a small biotech company with limited resources, we rely on visionary supporters and collaborators—academic scientists, advocacy groups, patients and forward-thinking insurance companies—to help us accelerate:
![]() Critical Research & Development – Advancing antiviral therapy and proving its efficacy.
Critical Research & Development – Advancing antiviral therapy and proving its efficacy.
![]() Rapid Prototyping & Clinical Trials – Ensuring accessibility and scalability of this life-saving technology.
Rapid Prototyping & Clinical Trials – Ensuring accessibility and scalability of this life-saving technology.
![]() Global Pandemic Preparedness – Creating an antiviral solution that protects against future outbreaks.
Global Pandemic Preparedness – Creating an antiviral solution that protects against future outbreaks.
