Preclinical evidence
The reverse-genetic engineered drug candidate, IBDV-R903/78 was exhaustively characterized. Importantly, the cytokine induction profile of IBDV-R903/78 improves the therapeutic index and IBDV-R903/78 can be effectively administered despite the presence of neutralizing antibodies.
The particular cytokine induction profile of reverse engineered IBDV R903/78 separates antiviral efficacy from inflammation improving the therapeutic index
Effective multiple oral administration of reverse engineered IBDV-R903/78 in mice in the presence of neutralizing antibodies.
Balb/C mice were dosed with multiple oral delivery of 1.7×106 IU of IBDV-R903/78 virus at days 0, 3, 7, 13, and 20. Necropsy was performed on days 1, 4, 8, 14, and 21. Tissue RNA was quantified by quantitative real-time RT-PCR. The transduction efficiency is expressed as IBDV copy numbers per mg of tissue.
Manufacturing
We have solved the problem of reproducible production
With reverse engineering technology a conventionally produced vaccine virus was recreated as the artificial IBDV-R903/78 virus. The two viruses have identical genomes, but the later can be manufactured without any concerns of labor intensive re-plaquing in case of the appearance of contaminant quasispecies or interfering small plaque mutant viruses, which is especially important if the virus is to be used in human therapy. Furthermore, our partner, ProBioGen AG, solved the problem of scalable manufacturing of IBDV-R903/78 drug candidate using a proprietary cell line enabling the production of millions of doses.
Importantly, our attenuated vaccine virus-based drug candidate is easily administered orally.
Clinical Evidence
IBDV has been proven clinically safe and effective against five different viruses in acute and chronic diseases. Therefore, IBDV could belong to the highly select 10% class of successful drug development cases.
Lead Clinical Option for Rapid Registration
A small, short ‘add-on’ herpes zoster Phase I/II trial could pave the way for fast regulatory approval.
In a severe herpes zoster ophthalmicus (HZO) infection, by complementing conventional acyclovir therapy with an add-on oral IBDV immunostimulatory treatment, it was demonstrated that the healing times could be reduced into a few days.
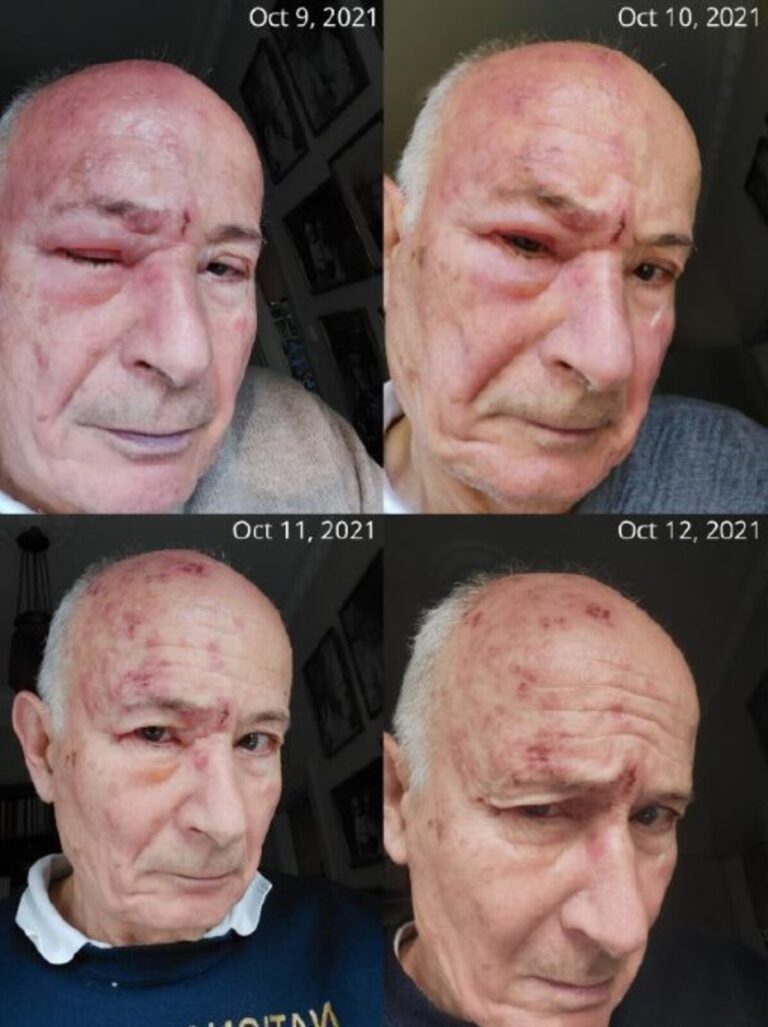
A picture is worth a thousand words
The CSO of HepC Inc’s HZO with orbital edema at the peak of disease and in recovery. The selfie pictures were taken between October 9, 2021, and October 12, 2021. Consent to the publication of patient information was granted by Tibor Bakacs, M.D., Ph.D., D.Sc., as he was the patient and the treating physician in his autobiography.
Regulatory support from the German Paul Ehrlich Institute and the USA NIH-ACTIV
Paul Ehrlich Institute
The PEI evaluated our IBDV program for the second time in 2020 and agreed that the data presented is adequate to support an oral delivery Phase I COVID-19 clinical trial. An excerpt from the advisory meeting with the experts of Paul Ehrlich Institute is copied below (the full briefing document and the minutes of the meeting are available upon request).

Question 4:
Does PEI agree that the GLP non-clinical toxicology studies conducted to date and reviewed in the briefing document are adequate to support the proposed Phase I trial of R903/78 for COVID-19?
PEI Response:
PEI agreed that the data presented is adequate to support a delivery Phase I clinical trial.
USA NIH-ACTIV
The ACTIV Agent Prioritization Team has thoroughly reviewed and evaluated Birnavirus/IBDV strain R903/78 against a set of pre-defined criteria, including rationale of use for treating COVID-19 or its resulting symptoms, mechanism of action, safety, and relevant preclinical and clinical data. Upon careful deliberation, the team felt that Birnavirus/IBDV strain R903/78 shows merit as a potential treatment for COVID-19. Quoted from the email of Joseph P. Menetski, PhD Associate Vice President Research Partnerships Foundation for the National Institutes of Health, Inc. Bethesda, MD 20852.